Abstract
Background: Primary/Post-Essential/Post-Polycythemic Myelofibrosis (MF) is characterized by impaired maturation of GATA1 deficient megakaryocytes, which we hypothesize directly contribute to disease. (J Clin Invest 2017). The selective AURKA inhibitor alisertib (MLN8237) was shown to promote megakaryocytic maturation, amelioration of bone marrow fibrosis and lessen disease in animal models (Nat Med 2015).
Methods: In an investigator sponsored multi-center pilot study (clinical trials.gov Identifier NCT 02530619), alisertib was administered to MF patients with at least DIPSS intermediate 1 risk who were in need of treatment, and were intolerant/refractory to or unlikely to benefit from JAK inhibitor therapy. Eligibility criteria also included an absolute neutrophil count ≥ 1 x109/L and platelet count ≥ 50 x109/L. Alisertib was provided by Takeda and administered at a dose of 50 mg by mouth twice daily for 7 days every three weeks. Adverse events (AEs) were monitored by common terminology criteria (Version 4.0). Laboratory correlative studies included assessment of the effects of alisertib and ruxolitinib on diagnostic samples and evaluation of changes in megakaryocytes and GATA1 expression in the bone marrow with therapy.
Results:
i) Patient characteristics
As of June 2017, 19 patients (63% males; median age 72 years) have been enrolled, including 68% primary MF and 32% post PV/ET MF; 26% were DIPSS high and 74% intermediate-risk.
11 (89%) patients had abnormal karyotype, including 7(64%) with unfavorable risk. Driver mutational frequencies were JAK2 58%, CALR 32%, and MPL 10%. Prior treatments included JAK inhibitors in 68% of patients.
13 (68%) patients remain on treatment (median number of cycles=4; range 1-16). Median follow up of patients that were alive was 5 months (range; 1-10 months).
ii) Safety data
Treatment related grade 4 hematological AEs included neutropenia, lymphopenia and thrombocytopenia seen in one patient each (5%). Grade 3 related hematological AEs included lymphopenia (n=4; 21%), anemia (n=3, 16%), febrile neutropenia (n=2, 11%), thrombocytopenia (n=1; 5%). The incidence of grade 3 related non- hematological AEs was as follows- vertigo (n=1; 5%), diarrhea (n=1; 5%); elevated lipase, alanine aminotransferase, and creatinine in one patient (5%) each. There was one unrelated grade 5 event -pneumonia with renal failure.
iii)Preliminary efficacy data
a) Spleen response : 11(58%) patients had palpable splenomegaly ≥ 5 cm at time of study entry; 3 (27%) achieved ≥ 50% reduction in palpable spleen size.
b) Erythroid response: 8 (62%) patients were transfusion dependent at baseline, of which none achieved transfusion independence. One patient displayed a durable rise in hemoglobin of ≥ 1g/dl.
c) Leukocytosis/Thrombocytosis/Leukoerythroblastosis/LDH responses: 6 (32%) patients presented with leukocytosis and all (100%) had resolution of leukocytosis with treatment. Two patients (11%) had baseline thrombocytosis and both displayed ≥ 50% reduction in platelet count. Ten patients (53%) demonstrated leukoerythroblastosis and 2 (20%) displayed ≥ 50% improvement. All 19 patients had increased serum lactate dehydrogenase levels at time of study entry and 6 (32%) displayed ≥ 50% reduction with treatment.
d) Symptom response: Of the 9 patients with constitutional symptoms at presentation, 5 (56%) reported improvement/resolution of symptoms. Moreover, 4 of 16 (25%) patients with at least moderate symptom burden achieved ≥ 50% reduction in the MF-SAF score.
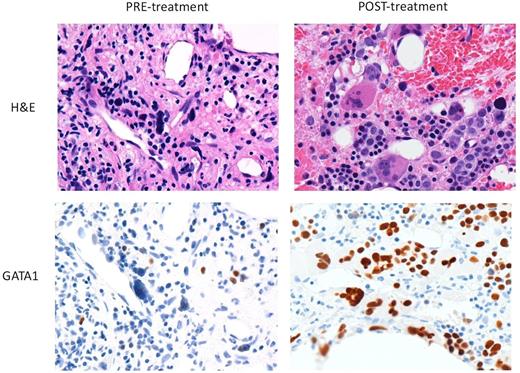
iv) Correlative studies: Alisertib, but not ruxolitinib, robustly promoted polyploidization and apoptosis of megakaryocytes derived from patient CD34+ cells at diagnosis. Morphological analysis of bone marrow revealed that alisertib led to improved megakaryocyte morphology and restoration of GATA1 staining (Figure 1).
Conclusions: In this study we have reproduced, in the setting of a clinical trial, our previous preclinical observations that alisertib appears to normalize megakaryopoiesis in the MPNs. The reduction in peripheral counts is consistent with studies that suggest atypical MF megakaryocytes promote expansion of leukocytes. Although Alisertib had limited activity beyond demonstrated myelosuppressive activity in patients treated an average of four cycles, it was relatively safe. Updated results including overall response assessment and bone marrow review will be presented.
Watts: Jazz Pharmaceuticals: Consultancy, Speakers Bureau. Altman: Syros: Consultancy; NCCN: Other: Educational speaker; BMS: Consultancy; Celgene: Consultancy; Astellas: Consultancy; Ceplene: Consultancy; Janssen Pharmaceuticals: Consultancy; Novartis: Consultancy; ASH: Other: Educational speaker. Giles: Novartis: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal